GABA Receptor
|
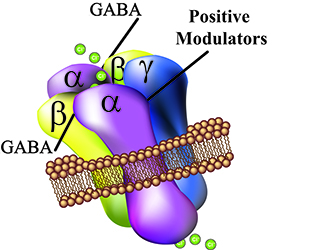
The GABA(A) receptors are membrane protein complexes that are composed of α, β, and γ subunits. The membrane receptors are majorily expressed in the brain but can also be found in different tissue such as muscle and immue cells. γ-aminobutyric acid (GABA) is the endogenous ligand for the GABA(A) receptors inhibiting the signaling transduction mediated by these receptors by hyperpolarization (increase of chloride ion efflux. Other molecules are known to modulate GABA(A) receptor actions such as benzodiazepines, alcohol, steroids and barbiturates. |
Our laboratory is using the IONFLUX (Fluxion) to carry out electrophysiological measurements. For the determination of GABA(A) receptor alpha subtype selectivity, we have generated HEK293 cell lines that express individual GABA(A) receptors. In addition, we are patch clamping other cell types such as leucocytes and investigate their behavior in the presence of new imidazobenzodiazepine compounds. The IONFLUX is a plate based instrument that allows us to execute more than 3000 data points a day focusing on the determination of compound dose dependencies. |
 Nina Yuan Nina Yuan |
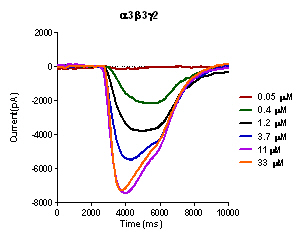 |
This is an example of current sweep of a α3β3γ2 GABA(A) receptor expressed in HEK293T cells in response to increasing concentations of GABA using the IONFLUX. GABA is applied to cells patched in two traps with 20 cells per trap. Using a 96 well plate eight experiments can be run simultaniously.
|
|
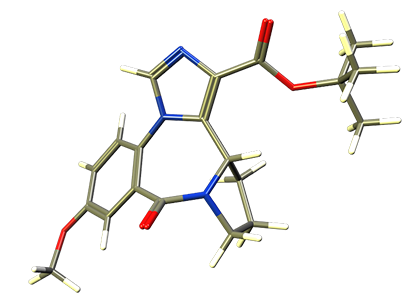
XHE-III-74 was the first α4 selective GABAA receptor modulator investigate by our collaborators and us in respect to asthma. Yocum et al showed airway smooth muscle relaxation of XHE-III-74 in mouse and human ex vivo preparation. It was also active as an aerosol reducing airway hyperresponsiveness. However, its different metabolic stability in mice and in human impeded the development of XHE-III-74 (Mol. Pharm. 2016). In addition, high levels of XHE-III-74 were observed in the brain, which caused CNS effects at dosages of 40 mg/kg and higher. |
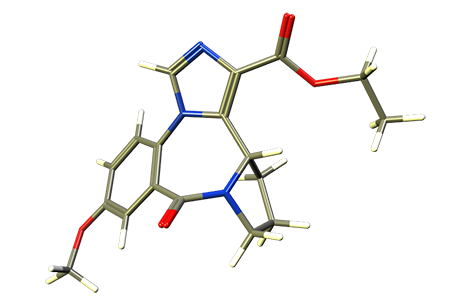
XHE-III-74EE is an alternative derivative of XHE-III-74, bearing an ethyl ester instead of a t-butyl ester. The compound was stable in presence of mouse or human liver microsomes. When given ip, XHE-III-74EE protected against airway hyperresponsiveness in a murine model of asthma (Mol. Pharm. 2016). It also attenuated smooth muscle relaxation ex vivo. In respect to inflammation, XHE-III-74EE reduced the expression of IL-2 in Jurkat cells and increased negative membrane potential in vitro. Unfortunately, XHE-III-74EE was not persistent in vivo with a short life time of 16 minutes in blood. Pronounced CNS effects were observed at dosages of 40 mg/kg ip and higher.
XHE-III-74EE is a
α4β3γ2 GABAA receptor selective compound.
XHE-III-74A was investigated due to metabolism of XHE-III-74EE to its corresponding acid. The compound is selective towards the α4β3γ2 GABAA receptor although less potent than XHE-III-74EE (Mol. Pharm. 2016). It did not cause any CNS effects and reduced eosinophilia in the mouse lung. In addition, it reduces the expression of IL-2 in Jurkat cells and significantly decreases the concentration of [Ca2+] in activated T-cells. XHE-III-74A relaxed precontracted tracheal rings ex vivo. However, the pharmacokinetics of XHE-III-74A are suboptimal with a half-life of 13 minutes in blood due to rapid excretion.
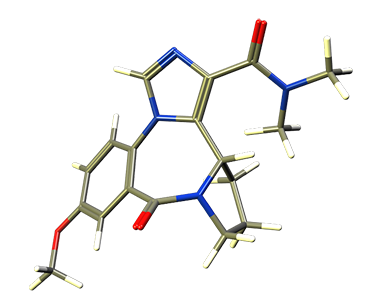
MRS-01-66, a dimethylamide analog of XHE-III-74, was shown to relax airway smooth muscle ex vivo and reduce airway hyperresponsiveness in a murine asthma model when given ip (EJMC 2017). Although MRS-01-66 is stable in the presence of mouse and human liver microsomes, it has inadequate pharmacokinetics. The half-life was less than 10 minutes and conversion to the mono methylamide but not the carboxylic acid was observed in vivo.
MRS-01-66 is selective towards the
α4β3γ2 GABAA receptor.
RJ-02-50 is a phenolic analog of XHE-III-74EE that has shown relaxation of airway smooth muscle and alleviated airway hyperresponsiveness in a murine model of asthma when given orally. In addition, it has anti-inflammatory properties reducing the number of CD4+ T-cells in bronchoalveolar lavage fluid. CD4+ T-cell directly responded to RJ-02-50 with an increase of negative current. High metabolic stability and long half-life was observed in vivo, making it an excellent drug candidate for asthma. The compounds was not detected in the brain and no CNS effects were observed using a rotarod study with a 100 mg/kg oral dose. RJ-02-50 is selective towards the α4β3γ2 GABAA receptor. SH-053-2’F-R-CH3 acid is an analog of alpha 5 selective GABAA receptor modulator SH-053-2’F-R-CH3 (AJPLCMP 2015). In contrast to SH-053-2’F-R-CH3, SH-053-2’F-R-CH3 acid did not crossing the BBB and therefore induced no CNS effect. It is stable in the presence of mouse and liver microsomes and has very good pharmacokinetic parameters. SH-053-2’F-R-CH3 acid relaxed airway smooth muscle and attenuated airway hyperresponsiveness in a murine model of asthma. SH-053-2’F-R-CH3 acid also relaxed precontracted human smooth muscle. Finally, the acid had also anti-inflammatory properties due to the reduction of eosinophils and leukocytes. Overall, this protic α5 selective GABAA receptor modulator is an excellent drug candidates for asthma.  |